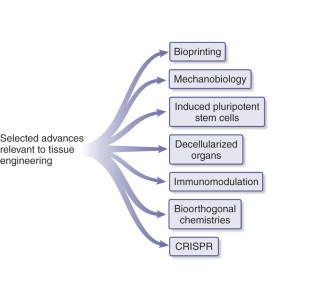
Tremendous progress has been achieved in the field of tissue engineering in the past decade. Several major challenges laid down 10 years ago, have been studied, including renewable cell sources, biomaterials with tunable properties, mitigation of host responses, and vascularization. Here we review advancements in these areas and envision directions of further development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
265,23 € per year
only 22,10 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Engineered biomaterials for in situ tissue regeneration
Article 06 July 2020
The stiffness of living tissues and its implications for tissue engineering
Article 21 February 2020
Ultrasound-assisted tissue engineering
Article 02 April 2024
References
- Langer, R. & Vacanti, J.P. Tissue engineering. Science260, 920–926 (1993). ArticleCASPubMedGoogle Scholar
- Khademhosseini, A., Vacanti, J.P. & Langer, R. Progress in tissue engineering. Sci. Am.300, 64–71 (2009). ArticleCASPubMedGoogle Scholar
- Khademhosseini, A. & Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials28, 5087–5092 (2007). ArticleCASPubMedGoogle Scholar
- Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature448, 313–317 (2007). ArticleCASPubMedGoogle Scholar
- Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science318, 1917–1920 (2007). ArticleCASPubMedGoogle Scholar
- Engler, A.J., Sen, S., Sweeney, H.L. & Discher, D.E. Matrix elasticity directs stem cell lineage specification 126, 677–689 (2006).
- DeForest, C.A., Polizzotti, B.D. & Anseth, K.S. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat. Mater.8, 659–664 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- DeForest, C.A. & Anseth, K.S. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem.3, 925–931 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Martino, M.M., Briquez, P.S., Ranga, A., Lutolf, M.P. & Hubbell, J.A. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc. Natl. Acad. Sci. USA110, 4563–4568 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Pakulska, M.M., Miersch, S. & Shoichet, M.S. Designer protein delivery: From natural to engineered affinity-controlled release systems. Science351, aac4750 (2016). ArticlePubMedCASGoogle Scholar
- Veiseh, O. et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater.14, 643–651 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Vegas, A.J. et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol.34, 345–352 (2016). ArticleCASPubMedPubMed CentralGoogle Scholar
- Langer, R. et al. Tissue engineering: biomedical applications. Tissue Eng.1, 151–161 (1995). ArticleCASPubMedGoogle Scholar
- Vacanti, J.P. & Langer, R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet354 (Suppl. 1): S32–S34 (1999). ArticleGoogle Scholar
- Qi, H. et al. DNA-directed self-assembly of shape-controlled hydrogels. Nat. Commun.4, 2275 (2013). ArticlePubMedCASGoogle Scholar
- Todhunter, M.E. et al. Programmed synthesis of three-dimensional tissues. Nat. Methods12, 975–981 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Cohen, D.L., Malone, E., Lipson, H. & Bonassar, L.J. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng.12, 1325–1335 (2006). ArticleCASPubMedGoogle Scholar
- Khalil, S., Nam, J. & Sun, W. Multi-nozzle deposition for construction of 3d biopolymer tissue scaffolds. Rapid Prototyping J.11, 9–17 (2005). ArticleGoogle Scholar
- Miller, J.S. et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater.11, 768–774 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kolesky, D.B. et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater.26, 3124–3130 (2014). ArticleCASPubMedGoogle Scholar
- Murphy, S.V. & Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol.32, 773–785 (2014). ArticleCASPubMedGoogle Scholar
- Colosi, C. et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv. Mater.28, 677–684 (2016). ArticleCASPubMedGoogle Scholar
- Ober, T.J., Foresti, D. & Lewis, J.A. Active mixing of complex fluids at the microscale. Proc. Natl. Acad. Sci. USA112, 12293–12298 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kang, H.-W. et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol.34, 312–319 (2016). ArticleCASPubMedGoogle Scholar
- Karp, J.M. & Leng Teo, G.S. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell4, 206–216 (2009). ArticleCASPubMedGoogle Scholar
- Ranganath, S.H., Levy, O., Inamdar, M.S. & Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell10, 244–258 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Sarkar, D. et al. Engineered cell homing. Blood118, e184–e191 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Levy, O. et al. mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood122, e23–e32 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Zuk, P.A. et al. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell13, 4279–4295 (2002). ArticleCASPubMedPubMed CentralGoogle Scholar
- De Coppi, P. et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol.25, 100–106 (2007). ArticleCASPubMedGoogle Scholar
- Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science337, 816–821 (2012). CASPubMedPubMed CentralGoogle Scholar
- Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science339, 819–823 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ran, F.A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc.8, 2281–2308 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell153, 910–918 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Servick, K. Gene-editing method revives hopes for transplanting pig organs into people. Science 10.1126/science.aad4700 (2015).
- Reardon, S. New life for pig-to-human transplants. Nature527, 152–154 (2015). ArticleCASPubMedGoogle Scholar
- Reardon, S. Gene-editing record smashed in pigs. Nature 10.1038/nature.2015.18525 (2015).
- Azagarsamy, M.A. & Anseth, K.S. Bioorthogonal click chemistry: An indispensable tool to create multifaceted cell culture scaffolds. ACS Macro Lett.2, 5–9 (2013). ArticleCASPubMedGoogle Scholar
- Sakiyama-Elbert, S.E. & Hubbell, J.A. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J. Control. Release69, 149–158 (2000). ArticleCASPubMedGoogle Scholar
- Sakiyama-Elbert, S.E. & Hubbell, J.A. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J. Control. Release65, 389–402 (2000). ArticleCASPubMedGoogle Scholar
- Swift, J. et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science341, 1240104 (2013). ArticlePubMedPubMed CentralCASGoogle Scholar
- Huebsch, N. et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater.9, 518–526 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Khetan, S. et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater.12, 458–465 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Yang, C., Tibbitt, M.W., Basta, L. & Anseth, K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater.13, 645–652 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Anderson, J.M., Rodriguez, A. & Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol.20, 86–100 (2008). ArticleCASPubMedGoogle Scholar
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials29, 2941–2953 (2008). ArticleCASPubMedGoogle Scholar
- Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol.25, 677–686 (2004). ArticleCASPubMedGoogle Scholar
- Porcheray, F. et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin. Exp. Immunol.142, 481–489 (2005). ArticleCASPubMedPubMed CentralGoogle Scholar
- Fishman, J.M. et al. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc. Natl. Acad. Sci. USA110, 14360–14365 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Spiller, K.L. et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials35, 4477–4488 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Spiller, K.L. et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials37, 194–207 (2015). ArticleCASPubMedGoogle Scholar
- Brown, B.N., Ratner, B.D., Goodman, S.B., Amar, S. & Badylak, S.F. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials33, 3792–3802 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Mokarram, N. & Bellamkonda, R.V. A perspective on immunomodulation and tissue repair. Ann. Biomed. Eng.42, 338–351 (2014). ArticlePubMedGoogle Scholar
- Du, Y., Lo, E., Ali, S. & Khademhosseini, A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc. Natl. Acad. Sci. USA105, 9522–9527 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Du, Y. et al. Surface-directed assembly of cell-laden microgels. Biotechnol. Bioeng.105, 655–662 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ke, Y., Ong, L.L., Shih, W.M. & Yin, P. Three-dimensional structures self-assembled from DNA bricks. Science338, 1177–1183 (2012). ArticleCASPubMedGoogle Scholar
- Wei, B., Dai, M. & Yin, P. Complex shapes self-assembled from single-stranded DNA tiles. Nature485, 623–626 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Park, A., Wu, B. & Griffith, L.G. Integration of surface modification and 3D fabrication techniques to prepare patterned poly(L-lactide) substrates allowing regionally selective cell adhesion. J. Biomater. Sci. Polym. Ed.9, 89–110 (1998). ArticleCASPubMedGoogle Scholar
- Giordano, R.A. et al. Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing. J. Biomater. Sci. Polym. Ed.8, 63–75 (1996). ArticleCASPubMedGoogle Scholar
- Vozzi, G., Flaim, C., Ahluwalia, A. & Bhatia, S. Fabrication of PLGA scaffolds using soft lithography and microsyringe deposition. Biomaterials24, 2533–2540 (2003). ArticleCASPubMedGoogle Scholar
- Wilson, W.C. Jr. & Boland, T. Cell and organ printing 1: protein and cell printers. Anat. Rec. A Discov. Mol. Cell. Evol. Biol.272, 491–496 (2003). ArticlePubMedGoogle Scholar
- Boland, T., Mironov, V., Gutowska, A., Roth, E.A. & Markwald, R.R. Cell and organ printing 2: fusion of cell aggregates in three-dimensional gels. Anat. Rec. A Discov. Mol. Cell. Evol. Biol.272, 497–502 (2003). ArticlePubMedGoogle Scholar
- Malda, J. et al. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater.25, 5011–5028 (2013). ArticleCASPubMedGoogle Scholar
- Bertassoni, L.E. et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip14, 2202–2211 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Lee, V.K. et al. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials35, 8092–8102 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kolesky, D.B., Homan, K.A., Skylar-Scott, M.A. & Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA113, 3179–3184 (2016). ArticleCASPubMedPubMed CentralGoogle Scholar
- Bhattacharjee, T. et al. Writing in the granular gel medium. Sci. Adv.1, e1500655 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Christensen, K. et al. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng.112, 1047–1055 (2015). ArticleCASPubMedGoogle Scholar
- Highley, C.B., Rodell, C.B. & Burdick, J.A. Direct 3d printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater.27, 5075–5079 (2015). ArticleCASPubMedGoogle Scholar
- Shim, J.-H., Lee, J.-S., Kim, J.Y. & Cho, D.-W.Bioprintingof a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system.. J. Micromech. Microeng.22, 085014 (2012). ArticleCASGoogle Scholar
- Tibbits, S. 4D printing: multi-material shape change. Architectural Design84, 116–121 (2014). ArticleGoogle Scholar
- Sydney Gladman, A., Matsumoto, E.A., Nuzzo, R.G., Mahadevan, L. & Lewis, J.A. Biomimetic 4D printing. Nat. Mater.15, 413–418 (2016). ArticleCASPubMedGoogle Scholar
- Badylak, S.F. The extracellular matrix as a scaffold for tissue reconstruction. in Seminars in Cell & Developmental Biology 377–383 (Elsevier, 2002).
- Ott, H.C. et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med.14, 213–221 (2008). ArticleCASPubMedGoogle Scholar
- Badylak, S.F., Taylor, D. & Uygun, K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng.13, 27–53 (2011). ArticleCASPubMedGoogle Scholar
- Song, J.J. & Ott, H.C. Organ engineering based on decellularized matrix scaffolds. Trends Mol. Med.17, 424–432 (2011). ArticleCASPubMedGoogle Scholar
- Arenas-Herrera, J.E., Ko, I.K., Atala, A. & Yoo, J.J. Decellularization for whole organ bioengineering. Biomed. Mater.8, 014106 (2013). ArticleCASPubMedGoogle Scholar
- Kaushal, S. et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat. Med.7, 1035–1040 (2001). ArticleCASPubMedPubMed CentralGoogle Scholar
- Amiel, G.E. et al. Engineering of blood vessels from acellular collagen matrices coated with human endothelial cells. Tissue Eng.12, 2355–2365 (2006). ArticleCASPubMedGoogle Scholar
- Zhang, W. et al. Elastomeric free-form blood vessels for interconnecting organs on chip systems. Lab Chip16, 1579–1586 (2016). ArticlePubMedPubMed CentralCASGoogle Scholar
- Lu, T.-Y. et al. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat. Commun.4, 2307 (2013). ArticlePubMedCASGoogle Scholar
- Ott, H.C. et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med.16, 927–933 (2010). ArticleCASPubMedGoogle Scholar
- Petersen, T.H. et al. Tissue-engineered lungs for in vivo implantation. Science329, 538–541 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Uygun, B.E. et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med.16, 814–820 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Baptista, P.M. et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology53, 604–617 (2011). ArticleCASPubMedGoogle Scholar
- Sullivan, D.C. et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials33, 7756–7764 (2012). ArticleCASPubMedGoogle Scholar
- Song, J.J. et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat. Med.19, 646–651 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Atala, A., Bauer, S.B., Soker, S., Yoo, J.J. & Retik, A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet367, 1241–1246 (2006). ArticlePubMedGoogle Scholar
- Goh, S.-K. et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials34, 6760–6772 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Teng, Y.D. et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. USA99, 3024–3029 (2002). ArticleCASPubMedPubMed CentralGoogle Scholar
- Niklason, L.E. et al. Functional arteries grown in vitro. Science284, 489–493 (1999). ArticleCASPubMedGoogle Scholar
- Lancaster, M.A. et al. Cerebral organoids model human brain development and microcephaly. Nature501, 373–379 (2013). ArticleCASPubMedGoogle Scholar
- Choi, S.H. et al. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature515, 274–278 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Huh, D., Hamilton, G.A. & Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol.21, 745–754 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Moraes, C., Mehta, G., Lesher-Perez, S.C. & Takayama, S. Organs-on-a-chip: a focus on compartmentalized microdevices. Ann. Biomed. Eng.40, 1211–1227 (2012). ArticlePubMedGoogle Scholar
- Wikswo, J.P. The relevance and potential roles of microphysiological systems in biology and medicine. Exp. Biol. Med.239, 1061–1072 (2014). ArticleCASGoogle Scholar
- Bhatia, S.N. & Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol.32, 760–772 (2014). ArticleCASPubMedGoogle Scholar
- Bhise, N.S. et al. Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release190, 82–93 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Zhang, Y.S. & Khademhosseini, A. Seeking the right context for evaluating nanomedicine: from tissue models in petri dishes to microfluidic organs-on-a-chip. Nanomedicine (Lond.)10, 685–688 (2015). ArticleCASGoogle Scholar
- Esch, E.W., Bahinski, A. & Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov.14, 248–260 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ingber, D.E. Reverse engineering human pathophysiology with organs-on-chips. Cell164, 1105–1109 (2016). ArticleCASPubMedGoogle Scholar
- Ebrahimkhani, M.R., Neiman, J.A., Raredon, M.S.B., Hughes, D.J. & Griffith, L.G. Bioreactor technologies to support liver function in vitro. Adv. Drug Deliv. Rev.69-70, 132–157 (2014). ArticleCASPubMedGoogle Scholar
- Huh, D. et al. Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc. Natl. Acad. Sci. USA104, 18886–18891 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Huh, D. et al. Reconstituting organ-level lung functions on a chip. Science328, 1662–1668 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wilmer, M.J. et al. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol.34, 156–170 (2016). ArticleCASPubMedGoogle Scholar
- Kim, S., Lee, H., Chung, M. & Jeon, N.L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip13, 1489–1500 (2013). ArticleCASPubMedGoogle Scholar
- Kim, H.J., Huh, D., Hamilton, G. & Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip12, 2165–2174 (2012). ArticleCASPubMedGoogle Scholar
- Torisawa, Y.-S. et al. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat. Methods11, 663–669 (2014). ArticleCASPubMedGoogle Scholar
- Nawroth, J.C. et al. A tissue-engineered jellyfish with biomimetic propulsion. Nat. Biotechnol.30, 792–797 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Shin, S.R. et al. Aligned carbon nanotube-based flexible gel substrates for engineering bio-hybrid tissue actuators. Adv. Funct. Mater.25, 4486–4495 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Raman, R. et al. Optogenetic skeletal muscle-powered adaptive biological machines. Proc. Natl. Acad. Sci. USA113, 3497–3502 (2016). ArticleCASPubMedPubMed CentralGoogle Scholar
- Menze, M.A. et al. Metabolic preconditioning of cells with AICAR-riboside: improved cryopreservation and cell-type specific impacts on energetics and proliferation. Cryobiology61, 79–88 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Heo, Y.S. et al. “Universal” vitrification of cells by ultra-fast cooling. Technology (Singap. World Sci.)3, 64–71 (2015). Google Scholar
- Bruinsma, B.G. et al. Supercooling preservation and transplantation of the rat liver. Nat. Protoc.10, 484–494 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
Author information
Authors and Affiliations
- Division of Biomedical Engineering, Department of Medicine, Biomaterials Innovation Research Center, Brigham and Women's Hospital, Harvard Medical School, Cambridge, Massachusetts, USA Ali Khademhosseini
- Harvard-MIT Division of Health Sciences and Technology, Cambridge, Massachusetts, USA Ali Khademhosseini & Robert Langer
- Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, Massachusetts, USA Ali Khademhosseini
- Department of Bioindustrial Technologies, College of Animal Bioscience and Technology, Konkuk University, Hwayang-dong, Gwangjin-gu, Seoul, Republic of Korea Ali Khademhosseini
- Department of Physics, King Abdulaziz University, Jeddah, Saudi Arabia Ali Khademhosseini
- David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA Robert Langer
- Department of Anesthesiology, Boston Children's Hospital, Boston, Massachusetts, USA Robert Langer
- Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA Robert Langer
- Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA Robert Langer
- Ali Khademhosseini







